Bladder cancer
Handling AEs of targeted therapies for metastatic UCa
Educational Objective: Get familiar with the current indications of immunotherapy and targeted therapies for metastatic urothelial carcinoma (UCa). Gain knowledge on how to handle adverse events (AEs) during and after immunotherapy, the FGFR inhibitor erdafitinib, and the antibody-drug conjugate enfortumab vedotin.
Specialty: Medical oncology, urology, clinical oncology
Target Audience: Specialists (CME: basic, intermediate), Residents (senior)
Latest update: September 2024
Background:
Regulatory approval status of drugs included in this topic (indications limited to the metastatic UCa setting, status 10 September 2024)
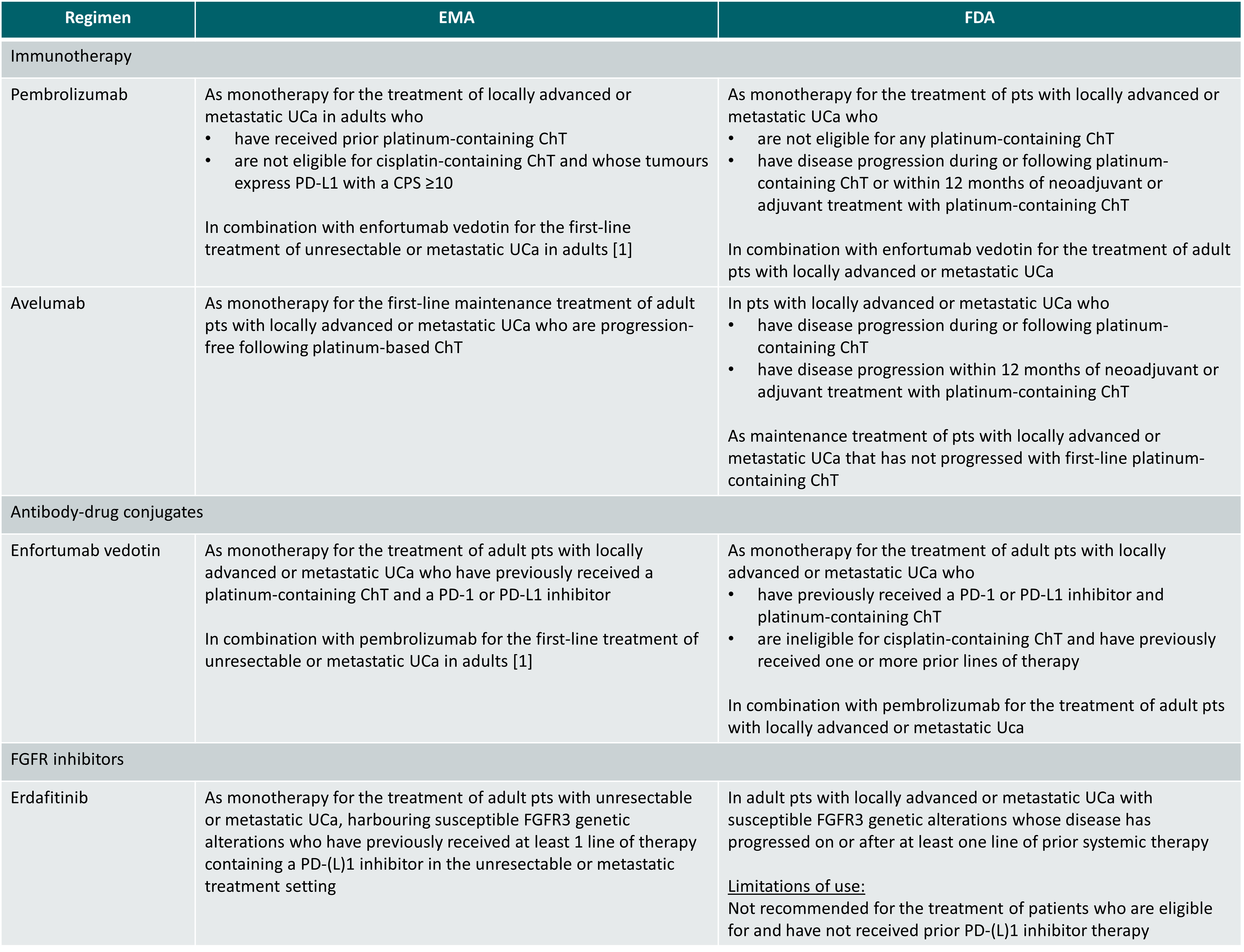
ChT: chemotherapy; CPS: combined positive score; PD-(L)1: programmed death-(ligand) 1
In all cases presented in this topic, it is assumed that the relevant antibodies/tests are used for PD-L1 and FGFR testing.
This educational platform includes case challenges with treatment options that may not be indicated for this use in your country. Please refer to your local prescribing information.
