Based on the clinical-pathological features, the benefit of adjuvant chemotherapy is uncertain for this young premenopausal patient with luminal B-like early breast cancer.
Guidelines
The decision on the adjuvant systemic treatment should be based on the patient’s risk of relapse (depending on tumour burden and tumour biology, e.g. grade, proliferation, lymphovascular invasion), the predicted sensitivity to particular types of treatment, the benefit from their use and their associated short- and long-term toxicities, the patient’s biological age, general health status, comorbidities and preferences [1].
The absolute benefit of adjuvant chemotherapy varies substantially and depends on the patient’s individual risk of relapse [1]:
- For luminal A-like tumours, which may be less chemo sensitive, endocrine therapy alone is recommended in the majority of cases. Chemotherapy should be considered if high disease burden is present.
- For luminal B-like tumours, which are likely to be more chemo sensitive, chemotherapy followed by endocrine therapy is recommended in the majority of cases. The use of chemotherapy next to endocrine therapy depends on the patient’s individual risk of recurrence, presumed responsiveness to endocrine therapy and patient preferences.
Several decision-making tools, based on clinical-pathological factors, are available to help predicting the recurrence risk and the potential benefit of systemic treatments (e.g. PREDICT Plus). In case of uncertainty on the benefit-risk ratio of adjuvant chemotherapy (after consideration of all clinical-pathological factors), gene expression profiling (GEP) can be used [1].
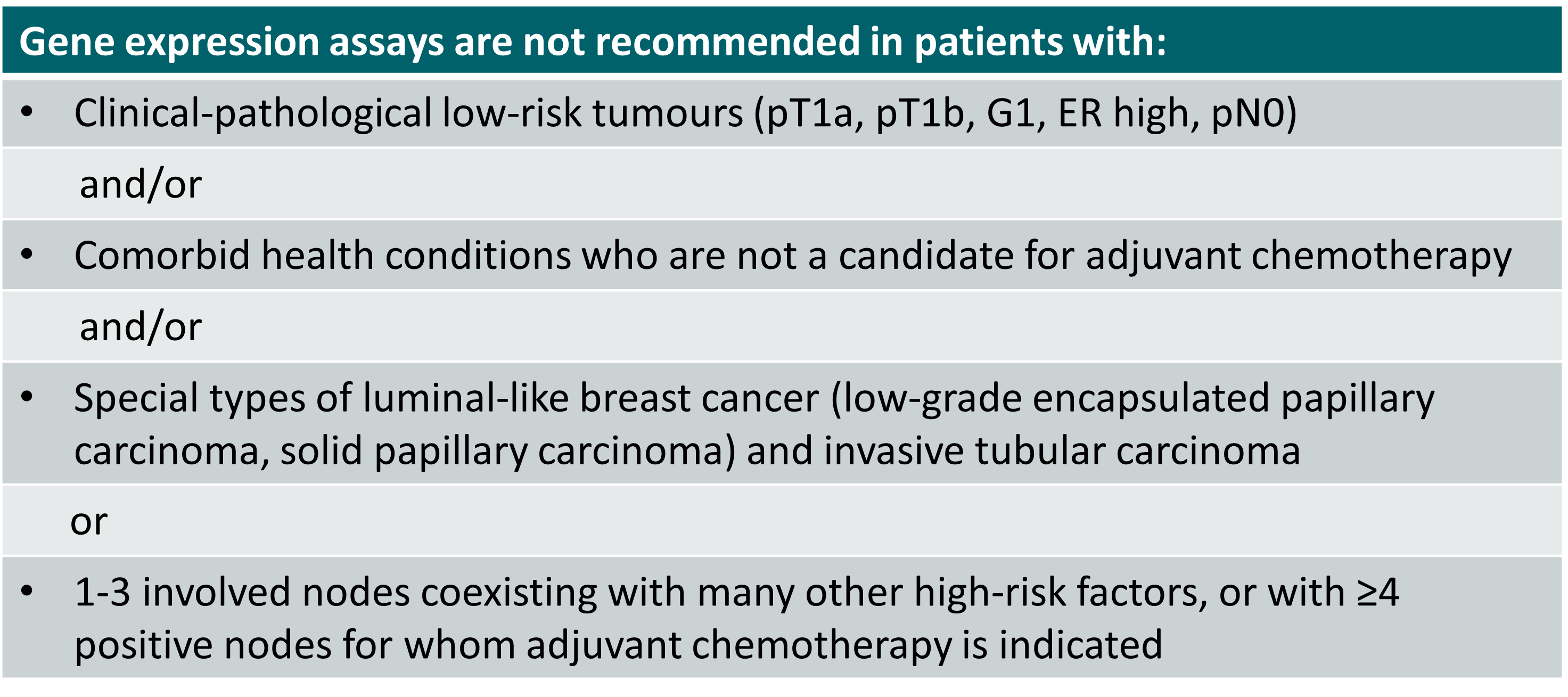
Cases for which use of GEP is not recommended [1]
Overview of GEP assays
Overview of GEP assays for consideration of adjuvant systemic therapy [2-12]
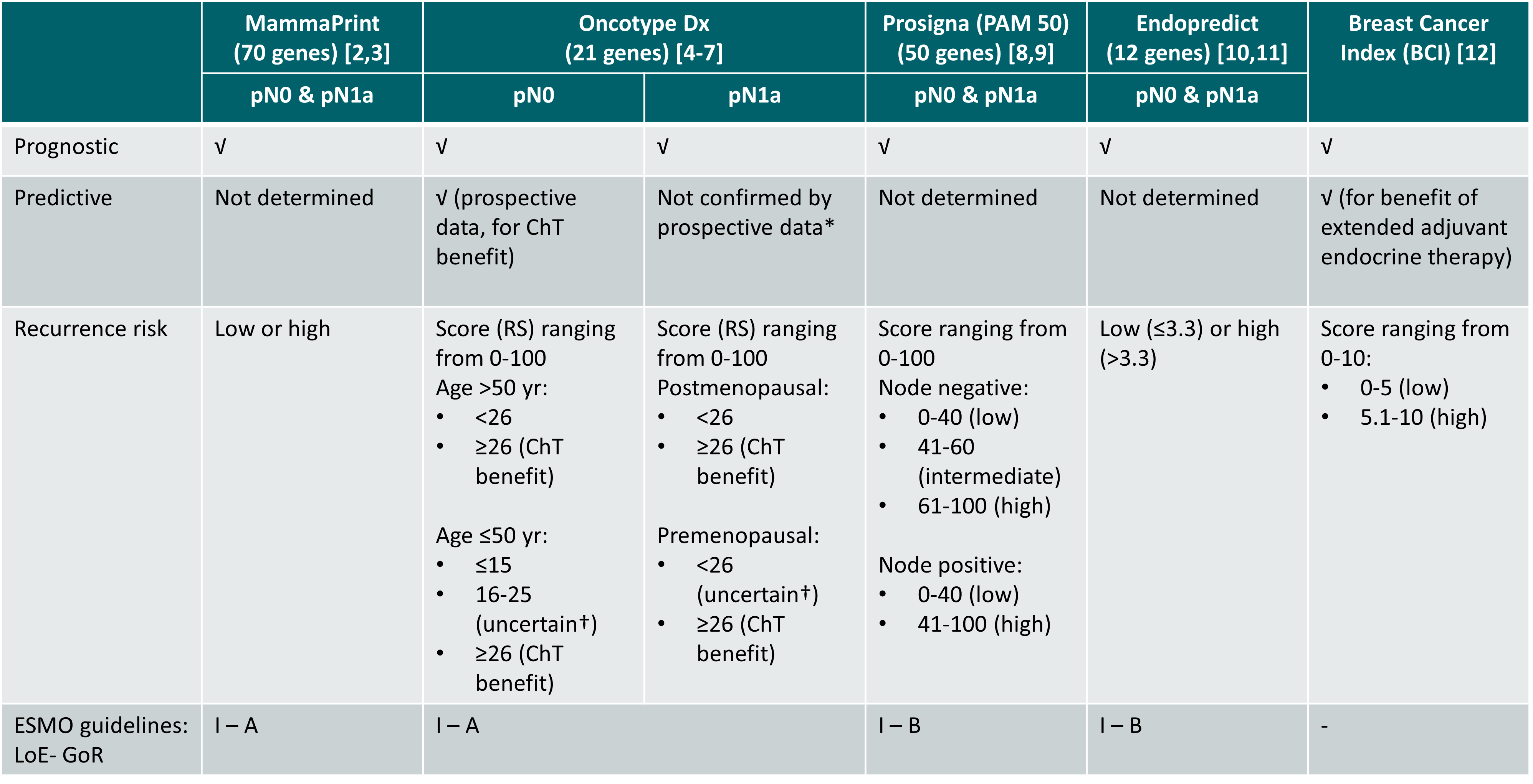
*Predictive value demonstrated in retrospectively analysed prospective study of node-positive, postmenopausal patients (SWOG 8814 [7]) but predictive value not confirmed by the prospective phase III RxPONDER study [6]
†Small to modest chemotherapy benefit but not sure if benefit was due to ovarian suppression effects promoted by chemotherapy. For this group, chemotherapy followed by endocrine therapy or OFS combined with either tamoxifen or AI can be considered
pN1a: 1-3 positive lymph nodes
ChT: chemotherapy; GoR: grade of recommendation; LoE: level of evidence; RS: recurrence score
It should be noted that GEP assays are not interchangeable and each assay provides different information for different patient populations [13,14].
Prospective studies in pN0 patients
Primary outcome of the phase III MINDACT and TAILORx studies [2-4]
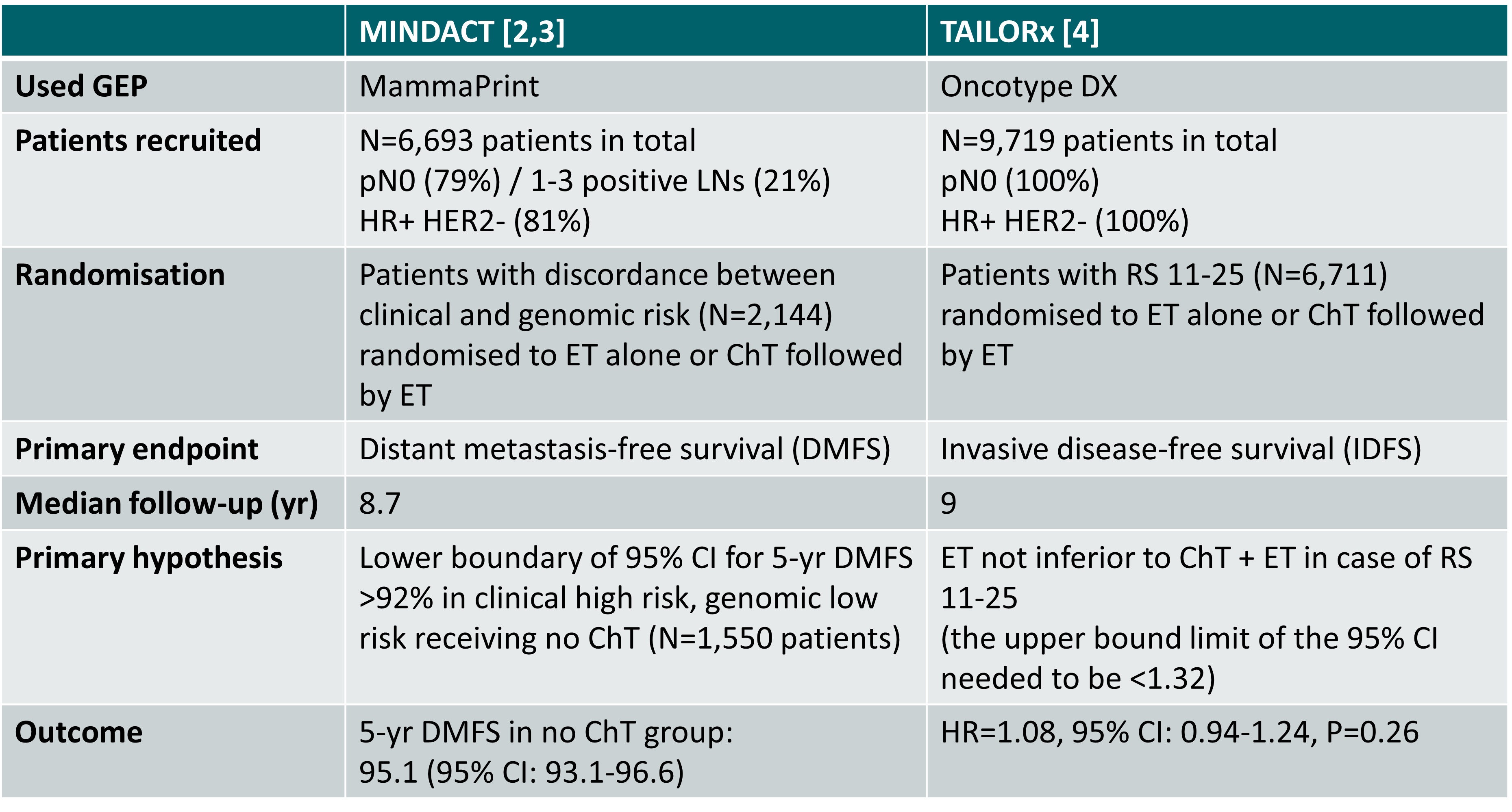
ChT: chemotherapy; ET: endocrine therapy; RS: recurrence score
An exploratory subgroup analysis of the MINDACT study showed that particularly in women >50 years old the omission of chemotherapy is safe (gain of 0.2% in distant metastasis-free survival at 8 years) [3]. The estimated gain in benefit of chemotherapy must be balanced with the treatment’s side effects.
Distant metastasis-free survival in the phase III MINDACT trial (median follow-up 8.7 years) [3]
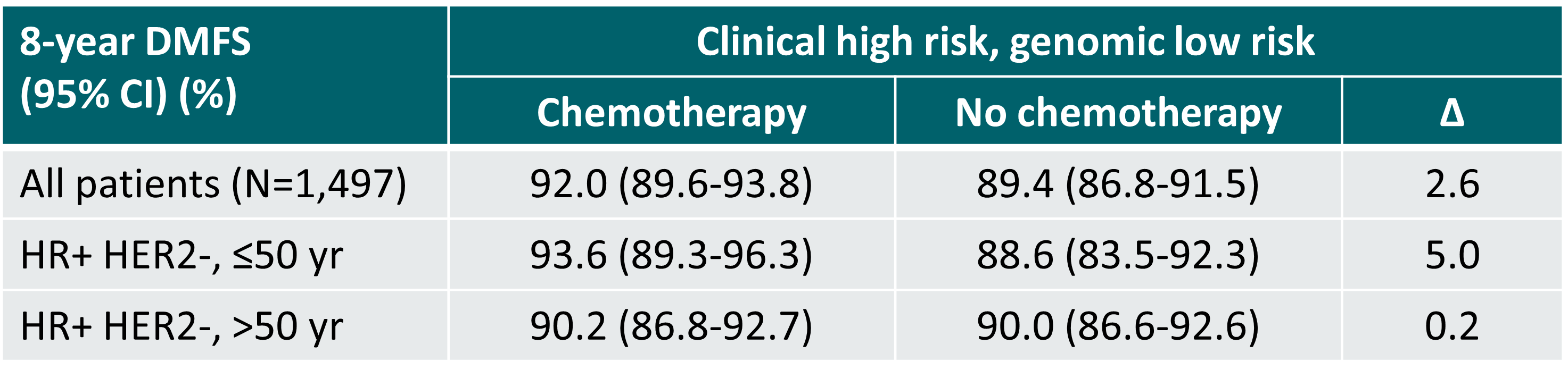
∆: absolute difference
In the TAILORx trial, the risk of distant recurrence at 9 years in women >50 years and having an intermediate recurrence score (RS 11-25) was similar irrespective of the use or non-use of chemotherapy, regardless of the clinical risk (high or low). In women ≤50 years and having an intermediate recurrence score, distant recurrence at 9 years was lower with the use of chemotherapy if clinical high risk. In case of clinical low risk, distant recurrence was similar irrespective of the use or non-use of chemotherapy [5].
Risk of distant recurrence at 9 years in women with a clinical high risk and intermediate recurrence score in the phase III TAILORx trial [5]
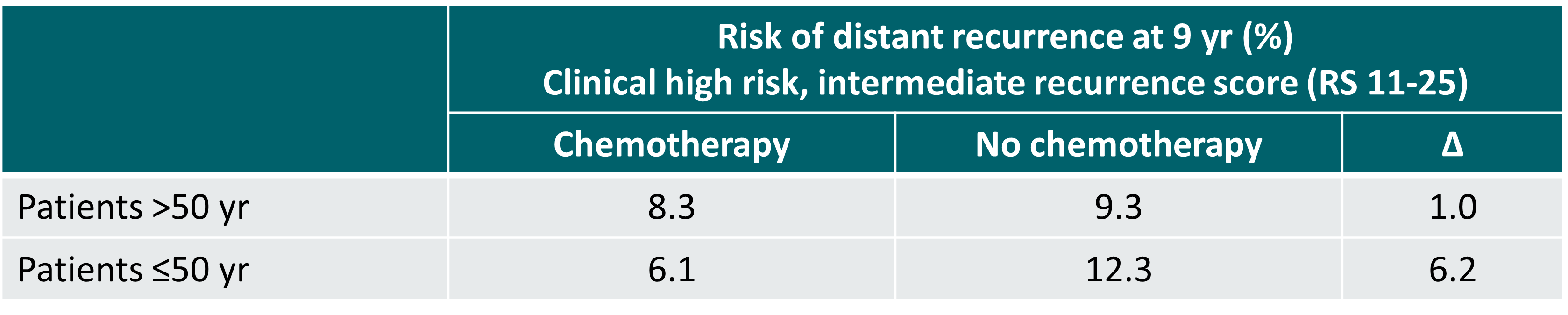
∆: absolute difference
An exploratory analysis of the TAILORx trial showed some benefit of chemotherapy in women ≤50 years with a recurrence score of 16-25 [5].





