Bladder cancer
Management of BCG-pretreated (very) high-risk NMIBC
The EAU guidelines define BCG failure as any high-grade (HG) disease occurring during or after BCG therapy (non-HG recurrences are NOT considered BCG failure) [1]. For the definition of BCG failure sub-categories, the EAU guidelines refer to the International Bladder Cancer Group consensus statement on clinical trial design for patients with BCG-exposed high-risk NMIBC [2].
EAU guidelines definition of BCG failure [1]
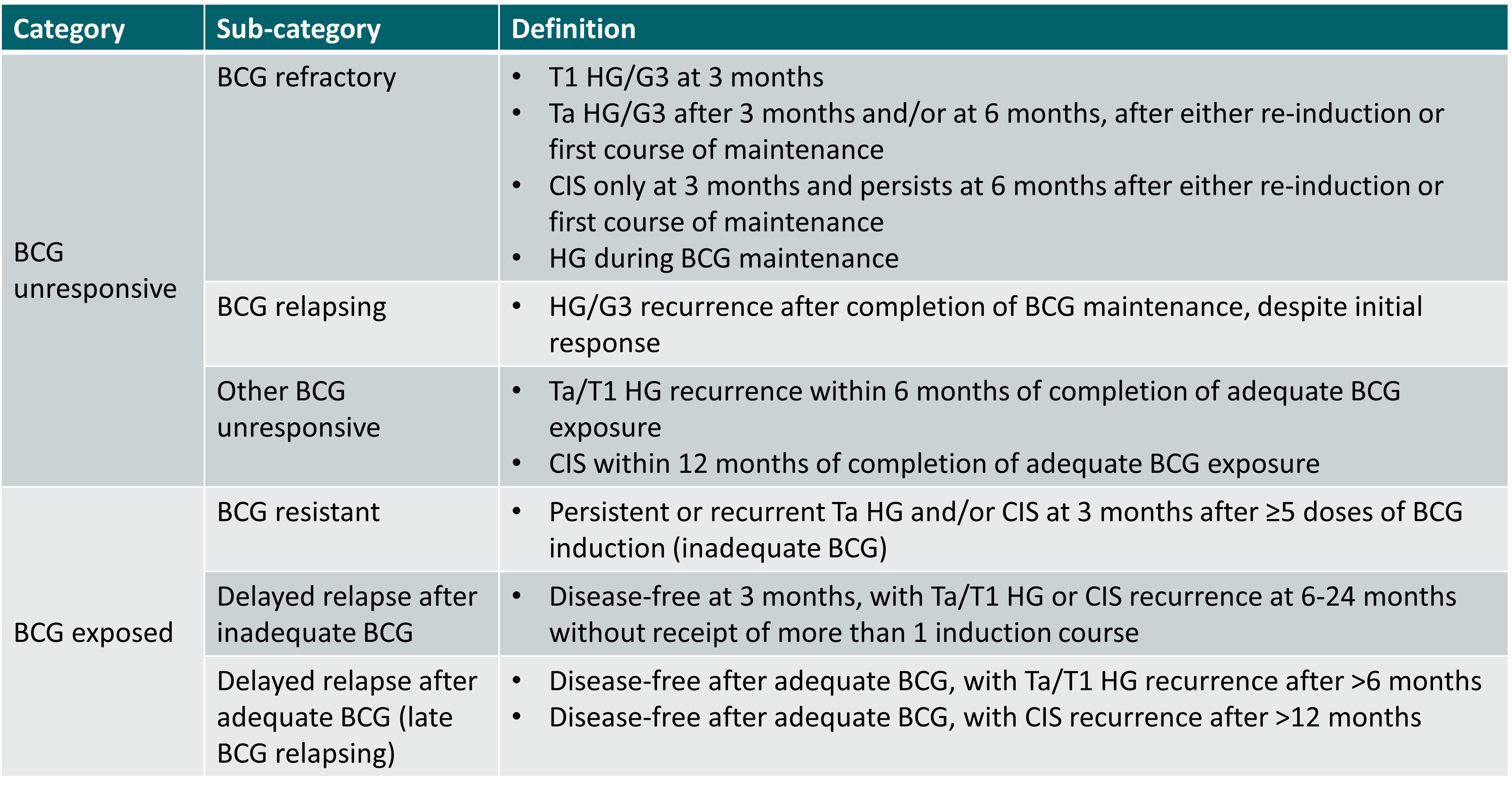
CIS: carcinoma in situ.
Adequate BCG treatment is defined as [1]:
- completion of at least 5 doses out of 6 of initial induction course and
- at least 2 doses out of 6 of second induction course or 2 doses out of 3 of maintenance therapy.
The NCCN guidelines refer to the recommendations on appropriate clinical trial designs in NMIBC by the International Bladder Cancer Group for the definition of BCG-unresponsive tumours [3,4]:
- BCG refractory if persistent HG disease at 6 months despite adequate BCG, including any stage or grade progression by 3 months after the first BCG cycle (i.e., HG T1 at 3 months after initial Ta, T1, HG disease, or CIS)
- BCG relapsing if HG disease after achieving a disease-free state at 6 months after adequate BCG.
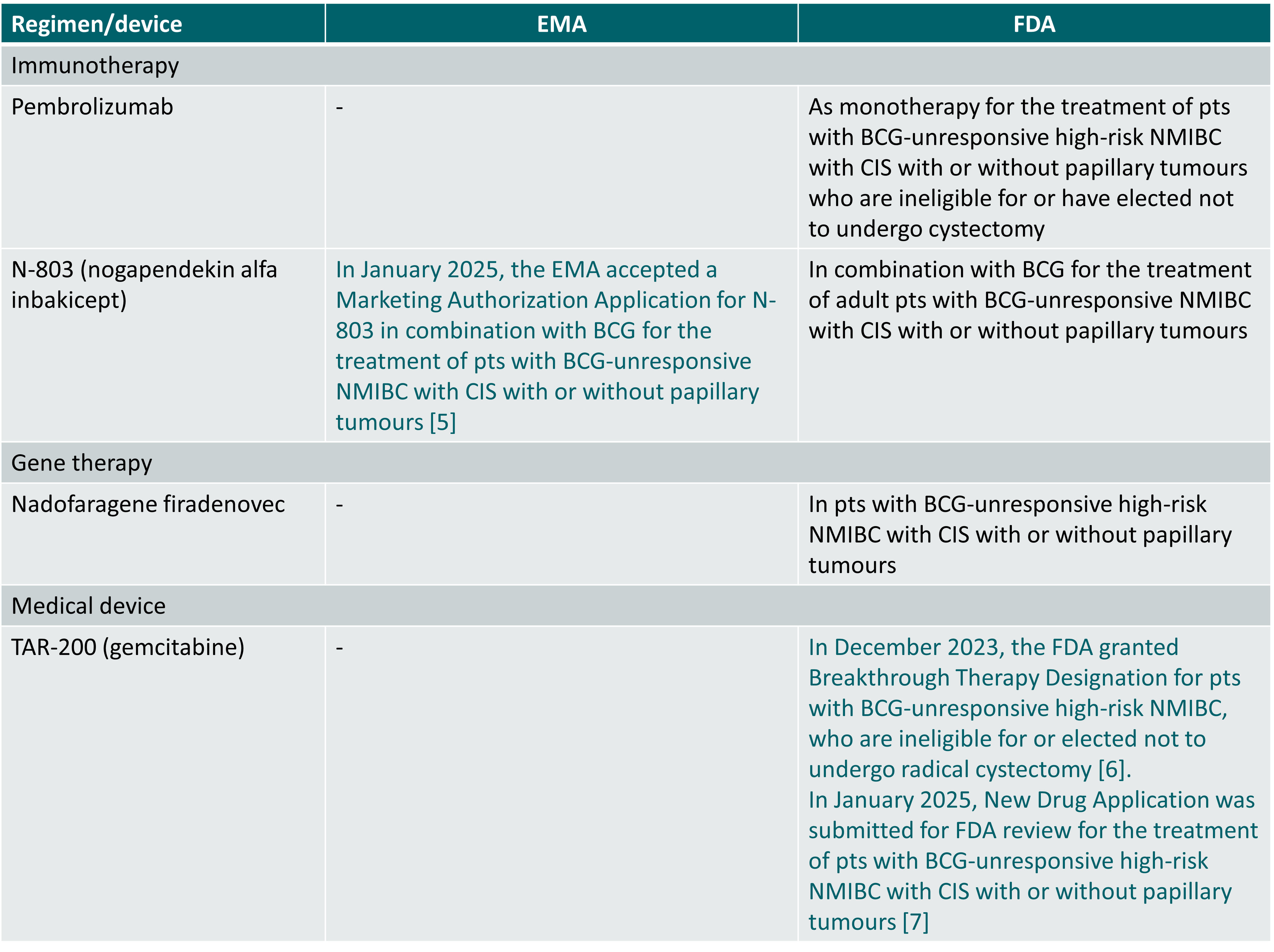
Regulatory approval status of drugs/medical devices included in this topic (indications limited to the NMIBC setting, status 12 February 2025)
- Gontero P, Birtle A, Compérat E, et al. European Association of Urology (EAU) guidelines on non-muscle-invasive bladder cancer (TaT1 and CIS). Update April 2024. Available at: https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/
- Roumiguié M, Kamat AM, Bivalacqua TJ, et al. Eur Urol 2022;82:34-46. PubMed
- Flaig TW, Spiess PE, Abern M, et al. National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology: bladder cancer. Version 6.2024. Available at: https://www.nccn.org/guidelines/category_1
- Kamat AM, Sylvester RJ, Böhle A, et al. J Clin Oncol 2016;34:1935-44. PubMed
- Press release of 27 January 2025 https://ir.immunitybio.com/news-releases/news-release-details/immunitybio-announces-european-medicines-agency-acceptance?field_nir_news_date_value%5bmin%5d=
- Press release of 4 December 2023 https://www.jnj.com/media-center/press-releases/johnson-johnsons-investigational-tar-200-granted-u-s-fda-breakthrough-therapy-designation-for-the-treatment-of-high-risk-non-muscle-invasive-bladder-cancer
- Press release of 15 January 2025 https://www.jnj.com/media-center/press-releases/new-drug-application-initiated-with-u-s-fda-for-tar-200-the-first-and-only-intravesical-drug-releasing-system-for-patients-with-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer
