Prostate cancer (US version)
Tailoring treatment for metastatic hormone-sensitive prostate cancer
Learning Objectives:
Upon completion of this ACCME-accredited CME program, the participants will be better able to:
1. identify novel therapies for the management of synchronous and metachronous mHSPC patients
2. select which patients will benefit from systemic treatment and which patients from local therapy
3. choose the most appropriate treatment/sequence of treatments for mHSPC patients and
4. assess the relevance and impact of recent data on mHSPC treatment
Educational Activity: Online Case-Based CME Program
Credits Offered: 1.00 AMA PRA Category 1 Credit(s)™
Release Date/End Date: 01/30/2023 - 02/15/2024
Estimated Run Time: 1 hour
Faculty/Panel Members:
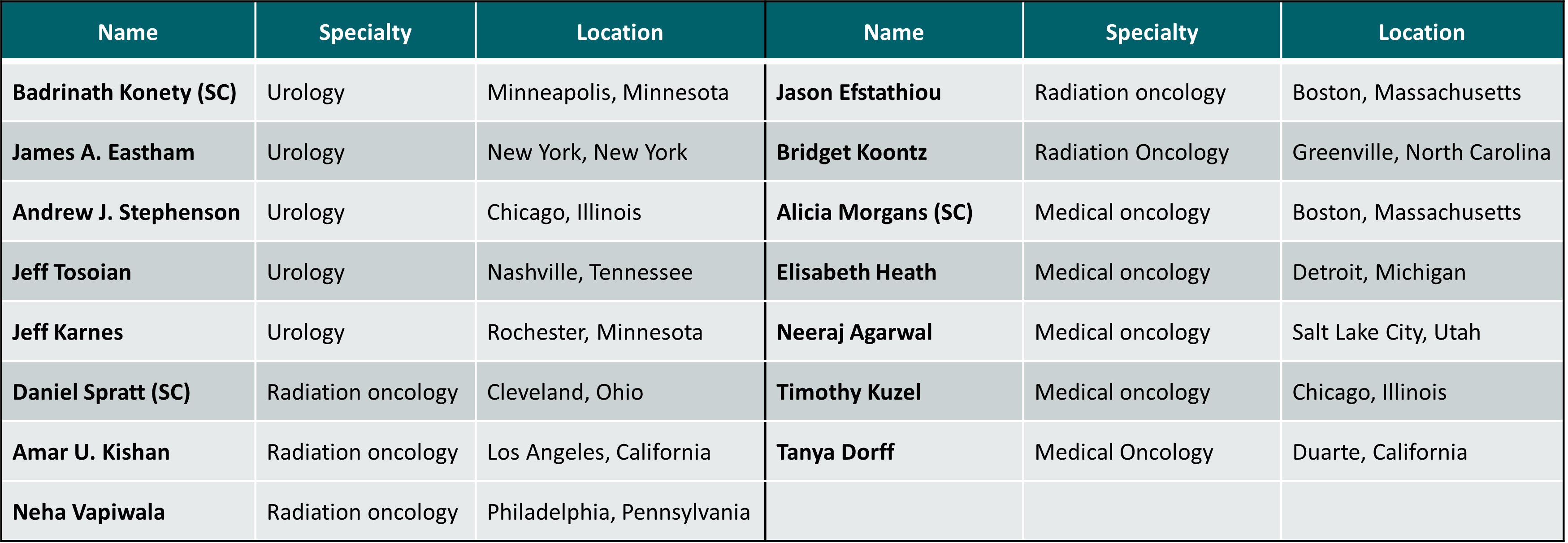
SC: Scientific Committee member.
Accreditation Statement: In support of improving patient care, Rush University Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), American Nurses Credentialing Center (ANCC), and the Accreditation Council for Pharmacy Education (ACPE), to provide continuing education for the healthcare team.
Designation Statement: Rush University Medical Center designates this internet enduring material for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only credit commensurate with the extent of their participation in the activity.
Disclosure Information: Rush University Medical Center has mechanisms in place to identify and resolve any potential conflicts of interest on the part of faculty and planners prior to the start of the activity. Individuals in control of content have disclosed the following:
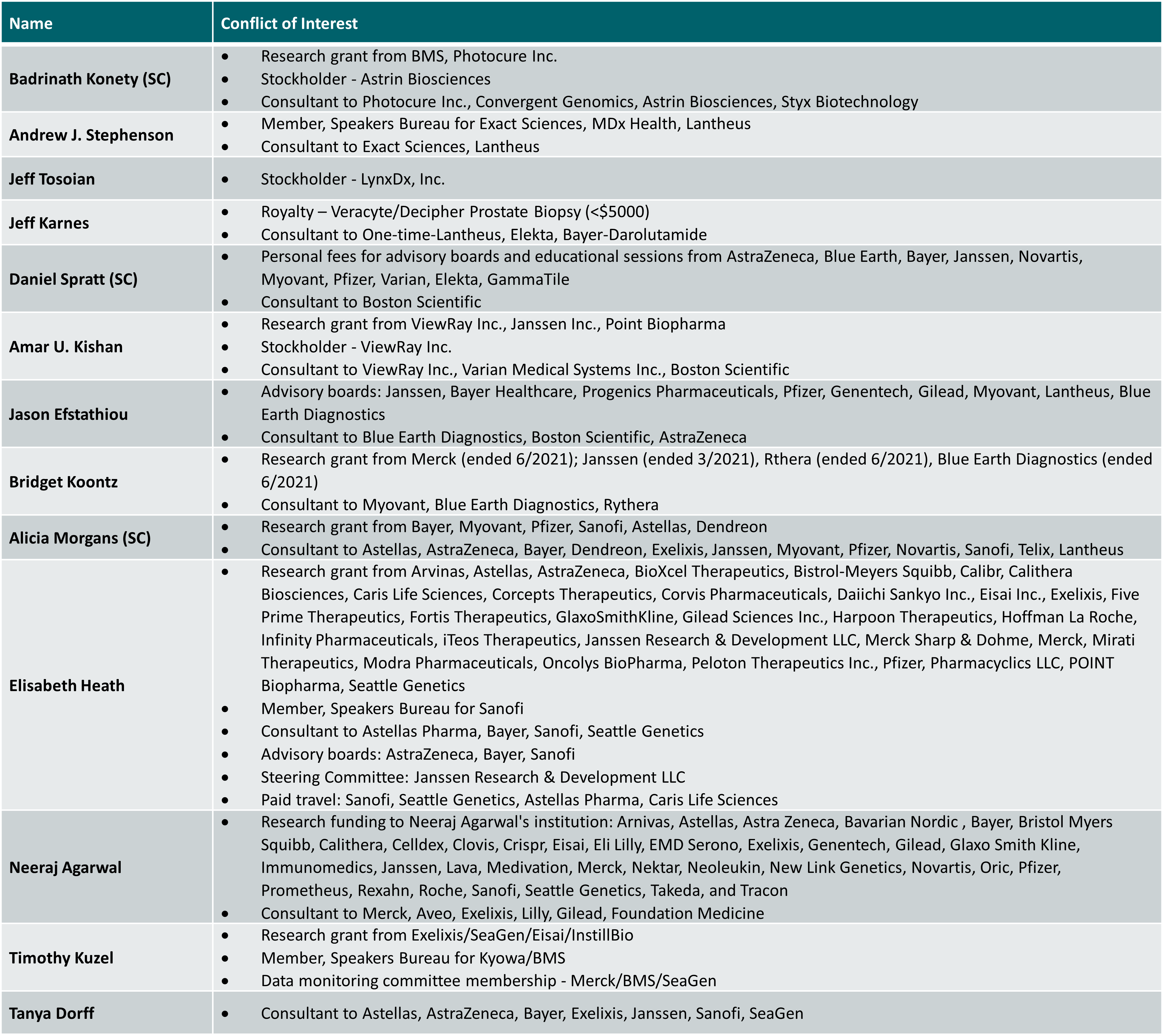
All of the relevant financial relationships listed for these individuals have been mitigated. The remaining faculty and reviewers of this activity have no relevant financial relationship(s) with ineligible companies to disclose.
Unapproved Uses of Drugs/Devices: In accordance with requirements of the FDA, the audience is advised that information presented in this continuing medical education activity may contain references to unlabeled or unapproved uses of drugs or devices. Please refer to the FDA approved package insert for each drug/device for full prescribing/utilization information.
Regulatory approval status of therapies included in this topic (status 1 December 2022)
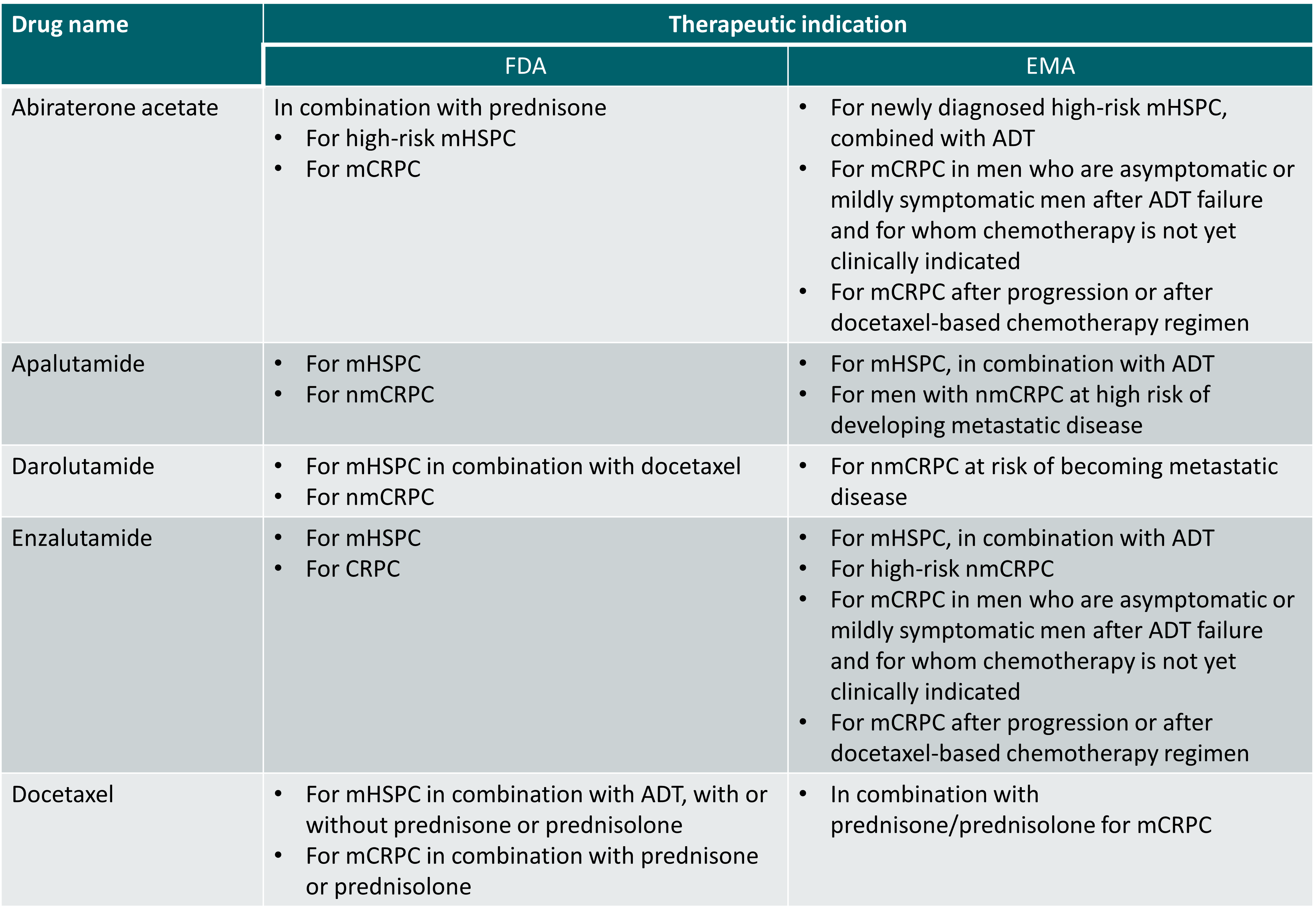
ADT: androgen deprivation therapy; CRPC: castration-resistant prostate cancer; mCRPC: metastatic castration-resistant prostate cancer; mHSPC: metastatic hormone-sensitive prostate cancer; nmCRPC: non metastatic castration-resistant prostate cancer.
This activity is funded in part by an unrestricted grant from Astellas.

